Periodic Table – Chemistry Study Notes: Periodic table is a tabular display of chemical elements arranged as per the increasing atomic number. In this blog, we will be discussing the periodic table for the benefit of SSC CGL aspirants. The same can be referred to for other competitive exams like SSC CHSL, RRB NTPC etc.
Periodic Table Definition
The periodic table is also known as the periodic table of chemical elements. The periodic table is based on the Periodic Law proposed by Mendeleev which states that properties of elements are periodic functions of their atomic masses.
Modern Periodic Table
The modern periodic table contains 118 elements. In the modern periodic table, the elements are arranged according to their atomic numbers. The periodic table is divided into groups and periods. The vertical rows are called groups and horizontal rows are called periods.
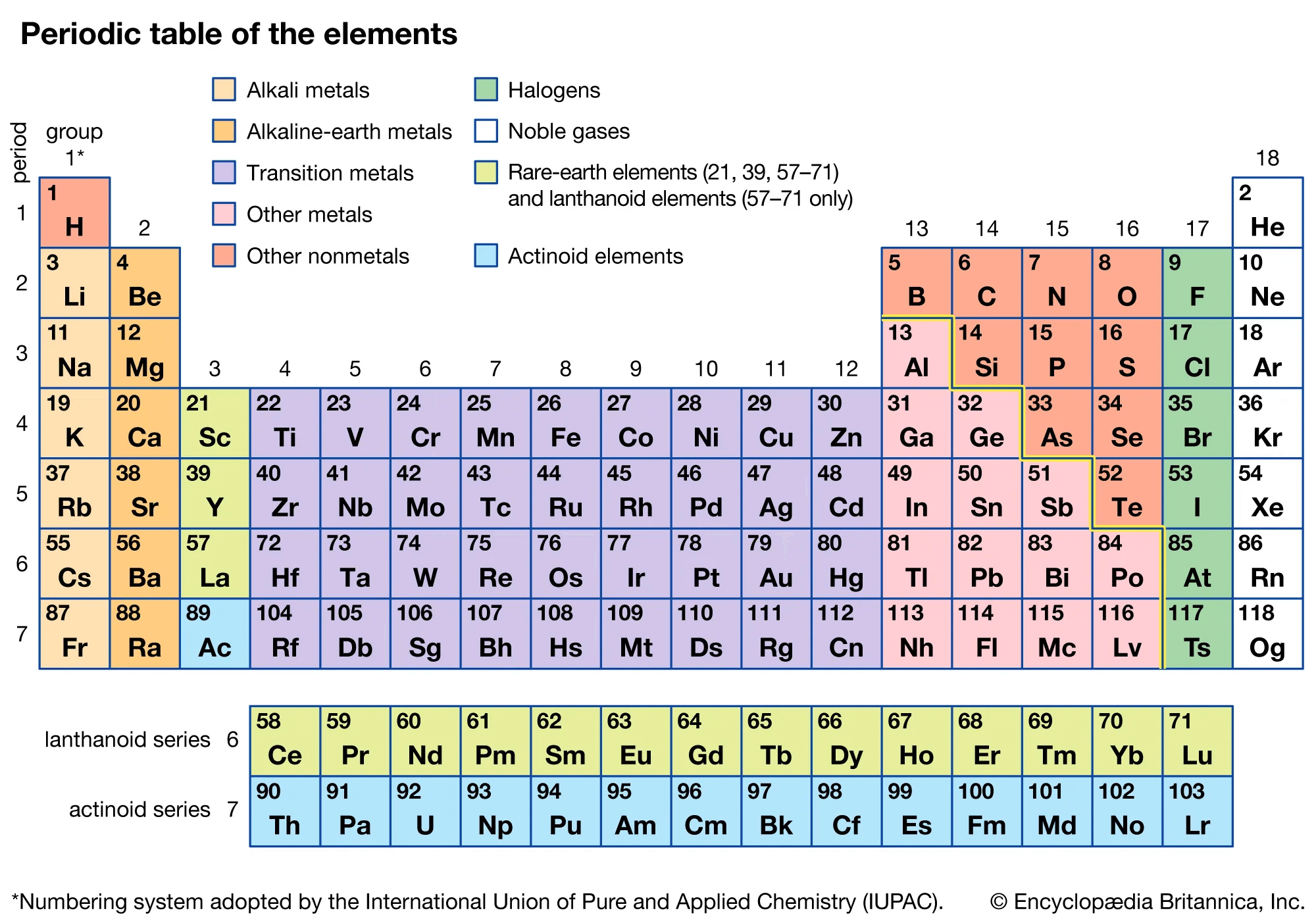
There are 7 periods and 18 groups in the modern periodic table. The periodic table is further divided into 4 blocks depending on the number of atomic orbitals that are filled with electrons. The four blocks are as follows:
- s-block
- p-block
- d-block
- f-block
s-block
- S-block contains two groups which are alkali metals and alkaline earth metals.
- Examples of alkali metals are Sodium, Pottasium etc.
- Examples of alkaline earth metals are Magnesium, Calcium etc.
p-block
- p-block consists of 6 groups belonging from group 13 to group 18
- Group 17 elements are called transition metals.
- Group 18 elements are called as noble gases or inert gases
Also read: Noble gases and their properties
d-block
- The d-block contains elements from group 3 to group 12
- There are 10 electrons in the d-orbital and therefore there are 10 groups in d-block
- The elements of group 3 to 12 are called as transition elements
- All the elements in the d-block are metals.
- Mercury is the only liquid metal.
f-block
- The f-block consists of lanthanides and actinides.
- Lanthanides are elements with atomic number 58 to 71.
- Actinides are elements with atomic number 90 to 103.
- All actinoids are radioactive in nature.
Conclusion
This was all about the periodic table which is a useful study material for the SSC CGL exam. For more updates, stay connected to Oliveboard.

Oliveboard is a learning & practice platform for premier entrance exams. We have helped over 1 crore users since 2012 with their Bank, SSC, Railways, Insurance, Teaching and other competitive Exams preparation.
Oliveboard Live Courses & Mock Test Series
- Monthly Current Affairs 2024
- Download RBI Grade B PYQ PDF
- Download IFSCA Grade A PYQs
- Download SEBI Grade A PYQs
- Attempt Free SSC CGL Mock Test 2024
- Attempt Free IBPS Mock Test 2024
- Attempt Free SSC CHSL Mock Test 2024
- Download Oliveboard App
- Follow Us on Google News for Latest Update
- Join Telegram Group for Latest Govt Jobs Update


